Answer:
66.79mL of the 21% NaCl solution are needed.
Step-by-step explanation:
1st) It is necessary to use the %m/v formula to calculate the grams of the 8.5%m/v solution of NaCl that will be in 165mL:
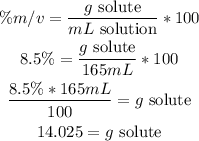
Now we know that we will need 14.025g of NaCl.
2nd) Using again the %m/v formula, we can calculate the volume of the 21% NaCl solution needed:

So, 66.79mL of the 21% NaCl solution are needed.