Answer:
a. 148.1 (g).
Step-by-step explanation:
What is given?
Volume of solution = 575.9 mL = 0.5759 L,
Molarity of solution = 1.6114 M,
Molar mass of CuSO4 = 159.5 g/mol.
Step-by-step solution:
To find the mass of CuSO4 in the solution, we have to use the molarity formula:
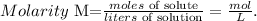
Let's solve for 'moles of solute', and replace the given data (remember that the volume must be in liters), like this:

The final step is to convert 0.928 moles of CuSO4 to grams using the molar mass of CuSO4, as follows:
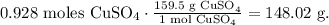
The answer would be the nearest to 148.02 g, which is a. 148.1 (g).