ANSWER
The pH is 7.302
Step-by-step explanation
Given that
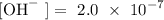
Follow the steps below to find the the pH
Step 1; Write the formula for calculating pOH
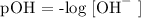
Step 2; Substitute the value of [OH^-] = 2.0 x 10 ^-7
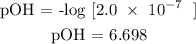
Recall, that pH + pOH = 14
Hence, we can find pH
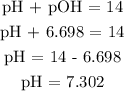
Therefore, the pH is 7.302