Step 1 - Finding the molar mass of NaAl(OH)CO3
To find the molar mass of a compound, we just have to multiply the molar mass of each element by the number of times it appears in the formula of the substance:
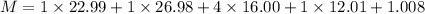
Working on the math, we obtain:
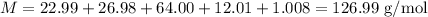
Step 2 - Finding the mass of one molecule of Rolaid
The number of Rolaid molecules in one mole is 6.02*10^23. Therefore, when we say the molar mass of Rolaid is 126.99 g/mol it is the same thing as saying that 6.02*10^23 molecules of Rolaid weight 126.99 g.
Therefore, to discover the mass of only one molecule of Rolaid, we should divide the molar mass by the number of molecules contained in one mole:
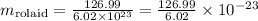
Working the math, we obtain thus:
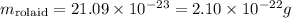
This is the mass of only one molecule of Rolaid.
Step 3 - Finding the mass of 3 molecules of rolaid
The hard part is done. Now that we have obtained the mass of one molecule of rolaid, we just have to multiply it by three in order to obtain the total mass of three molecules of rolaid:

The mass of three rolaid molecules is, therefore, 6.3*10^(-22) g.
Step 4