To answer this question, we have to use Boyle's Law:
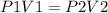
Where P1 is the initial pressure, V1 is the initial volume, P2 is the final pressure and V2 is the final volume.
We have to find V2 by solving the equation for this variable and replacing for the given values:
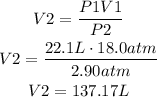
It means that the volume of oxygen when it is released is 137.17L.