1) Set the chemical equation.
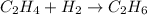
2) Calculating total bond energy
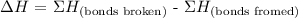
3) How many bonds were broken and formed?
One double bond between Carbons was broken, and the single bond between Hydrogens was broken.
Two single bonds between Carbon and Hydrogen were formed.
4) Calculating total bond energy
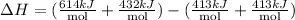
The total bond energy in the reaction is +220kJenergy