Answer
The final temperature in °C = 166.6 °C.
Step-by-step explanation
Given:
Initial temperature, T₁ = 20.0 °C
Initial volume, V₁ = 10.0 L
Final volume, V₂ = 15.0 L
What to find:
The final temperature in °C.
Step-by-step solution:
Since is held under isobaric conditions i.e constant pressure, the final temperature can be determined using the Charle's law equation below.
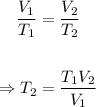
Plugging T₁ = (20.0 + 273.15) = 293.15 K, V₁ = 10.0 L and V₂ = 15.0 L into the equation, we have
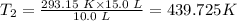
The final step is to convert the temperature from Kelvin to °C.
The final temperature = (439.725 - 273.15) = 166.6 °C.