Answer: the volume of the gas under the conditions given is 7.80 L
Step-by-step explanation:
The question requires us to determine the volume of a gas that contains 0.323 mol and is at 265 K and 0.900 atm.
We can apply the equation of ideal gases to solve this problem, as shown below:
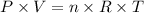
where P is the pressure of the gas (0.900 atm), V is the volume we want to calculate, n is the number of moles of gas (0.323 mol), R is the constant of gases and T is the temperature (265 K).
We can rearrange the equation to calculate the volume of the gas:
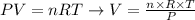
Since the pressure was given in atm and the temperature in K, we can use the following value for the constant of gases:
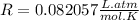
Note that the volume will be obtained in liters (L).
Applying the values provided by the question, we'll have:

Therefore, the volume of the gas under the conditions given is 7.80 L.