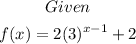
Part A: Domain of the function
Since the given function is an exponential function, then the domain is
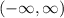
Part B: Range of the function
Since there is a constant 2 in the function, the range of the function is
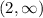
Part C: x-intercept
As the range starts at the horizontal asymptote of 2, then there is no x-intercept in the function
Part D: y-intercept
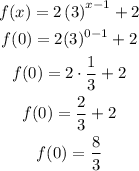
Therefore, the y-intercept is at (0,8/3).
Part E: Asymptote
Since the range starts at 2, the horizontal asymptote of the function is y = 2