Answer:
-79.14kJ
Step-by-step explanation:
1st) In the balanced reaction 791.4kJ are released when 2 moles of sulfur (S) react, so it is necessary to convert the 6.44g to moles, using the sulfur atomic mass (32g/mol):
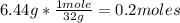
So, in this case, only 0.2 moles of sulfur will react.
2nd) Now, with a mathematical rule of three we can calculate the heat that will be released using 0.2 moles of S:
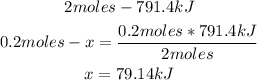
Finally, 79.14kJ will be released when 6.44g of S react with O2.