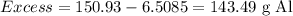Answer
143.49 g Al
Procedure
Using the following balanced equation determine the limiting reagent.
2AI(s) + Fe₂O₃(aq) --> AI₂O₃(aq) + 2Fe(s)
First, convert all the reagents to moles and then use the mole ratios method
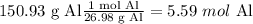
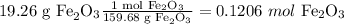
Molar ratios
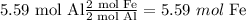
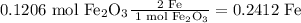
Therefore the limiting reagent is Fe₂O₃
Find the amount of remaining excess reactant by subtracting the mass of the excess reagent consumed from the total mass of excess reagent given.
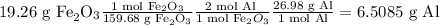
Excess = Mass of total excess reagent given – mass of excess reagent consumed in the reaction