Given,
The initial volume of the air, V₁=30 cm³=30×10⁻6 m³
The initial temperature of the air, T₁=29 °C=302.15 K
The final temperature of the air, T₂=4 °C=277.15 K
From Charles' law, we have,
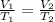
Where V₂ is the final volume of the air.
On substituting the known values,
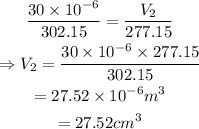
Thus the volume of the ai after reducing the temperature is 27.52 cm³