Answer:
The empirical formula for vanillin is C8H8O3.
Step-by-step explanation:
1st) It is necessary to calculate the amount of carbon (C), hydrogen (H) and oxygen (O) in the combusted products:
- From the 2.43g of CO2 we can find 1.77g of oxygen and 0.66g of carbon.
- From the 0.50g of water, we can find 0.05g of H and 0.44g of oxygen.
So, the total amount of carbon is 0.66g, of hydrogen is 0.05 and 2.21 of oxygen.
2nd) Now we have to calculate the percentage of each element in the 1.05g of sample, assuming that 1.05g is the 100%:
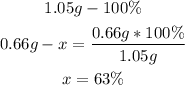
The percentage of carbon is 63%.
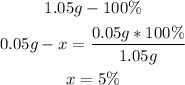
The percentage of hydrogen is 5%, and the rest (100%-63%-5%=32%) is oxygen.
3rd) Now we have to divide the percentages of each element between the atomic weight of them:
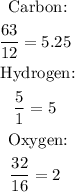
4th) Now we have to divide each value between the smallest number, in this case, 2:
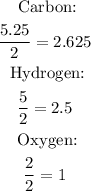
5th) As hydrogen and carbon gave a result nearly a 0.5 decimal, we must multiply them all by a number that converts it into the nearest integer, in this case we multiply by 3.
So, the empirical formula for vanillin is C8H8O3.