The heat Q absorbed by a sample with mass m of a material with specific heat c that increases its temperature by ΔT is:
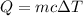
Find the change in temperature of the sample by subtracting the initial temperature from the final temperature:
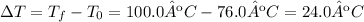
Replace m=1230g, c=4.68J/gºC and ΔT=24.0ºC to find the amount of heat added to the Lead:
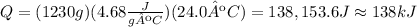
Therefore, the amount of heat added to the Lead was approximately 138kJ.