Answer:
23 j / g
Step-by-step explanation:
The latent heat of fusion is the amount of energy required to either melt or solidfiy a gram of a material at its melting point.
We know that 2.99 * 10^4 J are required to solidify 1.30 kg ( or 1300 g) of the metal; therefore, the latent heat is
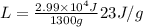
which is our answer!
Hence, the latent heat of fusion for the metal is 23 J/g.