(a). First, we need to identify the limiting reactant. Let's calculate the number of moles of each reactant:
For salicylic acid, we have that its molar mass is 138.1 g/mol (you can calculate it using the periodic table and doing the algebraic sum):
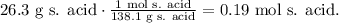
And now, let's see the number of moles of 17.7 g of acetic anhydride, where its molar mass is 102.1 g/mol:
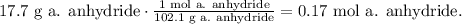
Now, you can realize that in the reaction 2 moles of salicylic acid react with 1 mol of acetic anhydride. Let's see how many moles of acetic anhydride requires to react with 0.19 moles of salicylic acid:
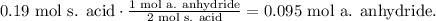
You can realize that we obtained less number of moles for acetic anhydride, meaning that the acetic anhydride is in excess and the salicylic acid is the limiting reactant, so we're going to work with this compound to find how many grams of aspirin is produced:
In the chemical equation, 2 moles of salicylic acid reacted produces 2 moles of aspirin, so the mole ratio between these two is 1:1 which is telling us that 0.19 moles of salicylic acid are producing 0.19 moles of aspirin. With this value, we can calculate the mass of aspirin as our theoretical yield. The molar mass of aspirin is 180.2 g/mol:
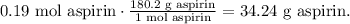
Remember that the theoretical yield is the amount that we expect to get in the reaction, in this case, is 34.24 grams of aspirin.
(b). Let's see the formula for percent yield:
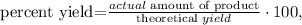
where the actual amount of product is what we obtained from the reaction, in this case, 30.7 grams of aspirin and the theoretical yield that we've already found (34.24 g). Replacing these values, we're going to have:
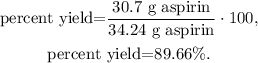
The percentage yield of the experiment is 89.66%.