We can find the number of moles using the molarity formula:
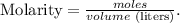
We have to convert 250 mL to L. Remember that 1 liter equals 1000 mL:
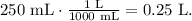
Now, that we have the value of volume in liters (0.25 L) and the molarity (0.0666 M (mol/L) ), we can clear in the formula for moles and replace the given values:
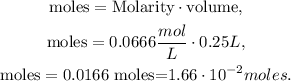
The answer is that we have 1.66 x 10^(-2) moles in 0.25 L of 0.0666 M of CH3CH2Cl.