We have the reaction equation:
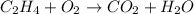
The balanced equation will be:
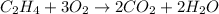
To have greater clarity of the bonds formed and broken we can write the blanced equation using the structural formula of molecules:
To know which bonds are formed and which are broken we look at both sides of the reaction. We compare the bonds we have in the reactants with the bond that we have in the products.
We have on the reactants side: 1 bond C=C, 4 C-H bonds and 3 O=O bonds
We have on the products side new bonds: 4 O=C bonds and 4 O-H bonds
So, we will have:
Broken links
1 bond C=C
4 bonds C-H
3 bonds O=O
The bonds forming are:
4 bonds C=O
4 bonds O-H