Answer:
0.0975 mol of solute (urea).
Step-by-step explanation:
Let's remember the formula of molality:
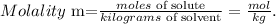
As we have the mass of the solvent (water) in g but not in kg, we have to convert it. Remember that 1 kg equals 1000 g:
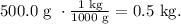
Now, let's solve for 'moles of solute' and replace the data that we have, like this:
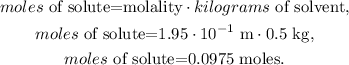
The answer would be that we have 0.0975 mol of solute (urea) in the solution.