To solve this problem we need to calculate the molar mas of Cu and CuO:
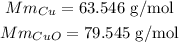
because of the chemical equation we know that 2 mols of Cu produce 2 mols of CuO. Now we have to convert those mols into mass, to do that we multiply the number of mols by the molar mass already calculated:
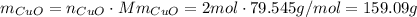
this means that for 2 mols of Cu that are consumed 159.09 g of CuO are produced. Then we just need to use this information to calculate the right proportion for 2.87mols of Cu. we can do that using a conversion factor:
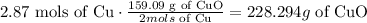
228.294 g of CuO are produced