ANSWER:
226.67 seconds
Explanation:
Given;
Mass (m) = 3.61 kg
T1 = 11.7 °C
T2 = 26.7 °C
Specific heat (C) = 4186 J/kg°C
Power (p) = 1000 W
We calculate the heat using the following formula:
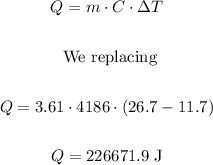
Now, we calculate the time using the following formula:
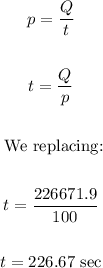
Therefore, it would take a total of 226.67 seconds