Answer:
0.1288g of KOH are needed.
Step-by-step explanation:
1st) It is necessary to write and balance the chemical reaction:

From the balanced reaction we know that 1 mole of KOH neutralizes 1 mole of HCl.
2nd) We have to calculate the number of HCl moles that are contained in 13.4mL of a 0.17M solution.
The molarity of the solution indicates that there are 0.17 moles of HCl in 1000mL of solution, so we can calculate the number of moles in 13.4mL of solution, using a mathematical rule of three:
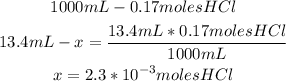
Now we know that there are 2.3x10^-3 moles of HCl in the solution.
3rd) From the stoichiometry of the reation, 1 mole of KOH neutralizes 1 mole of HCl. So, since the relation between KOH and HCl is 1:1, in this case, 2.3x10-3 moles of KOH are needed to neutralize the HCl.
Using the molar mass of KOH (56g/mol) we can convert the moles into grams:
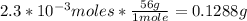
Finally, 0.1288g of KOH are needed.