We have by the table the relation of the concentration of antifreeze solution (ethylene glycol and water) with the boiling point. And they ask for the boiling point if the concentration it's just 20%.
If we look at the values of the concentration we realize that we have the value of the boiling point when the mixture is 10.5 concentrated and then when it is 29.1. We can use the interpolation method to approximate a value when the concentration is 20%.
We have the following data:
x1=10.5 y1=214
xd=20 yd=?
x2=29.1 y2=221
If we reply in the interpolation formula, we have:
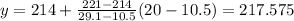
Then we can conclude that an approximation to the exact boiling point when we have a 20% mixture is 217.6°F