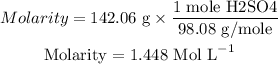Answer
1.448 Mol/L
Step-by-step explanation
We need to first calculate the mass of H2SO4 in 1.070 L as follows
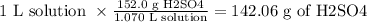
Now the molarity will be calculated as follows
Note: Molar mass of H2SO4 = 98.08 g/mol
H2SO4 = (2 x 1.00784) +32.065 + (4 x 15.999) = 98.08 g/mol