Step 1 - Reading the chemical equation
In order to correctly answer what the exercise is asking us, we first need to determine the moles proportion between the reactants.
This can be easily handled by "reading" the chemical equation. To do it, we just need to know that the bigger numbers, those that come before the formula of each substance, indicate its number of moles.
Therefore, for the given reaction:
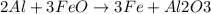
We can read it as:
2 moles of Al react with 3 moles of FeO thus producing 3 moles of Fe and one mole of Al2O3
Since the exercise is specifically asking us about the proportion between Al and FeO (Iron II oxide), we can further simplify the statement to:
2 moles of Al react with 3 moles of FeO
Step 2 - Using the proportion descibred by the reaction to find out how many moles of FeO are needed
Now that we have a "recipe" for the reaction, i.e., a fixed proportion between the reactants to work with, we can use it to predict the needed amount of FeO, in moles:
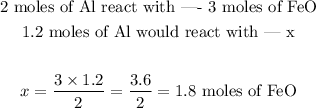
We would need thus 1.8 moles of FeO.