Given:
The mass of water is m = 1 gram
The water converts to steam at 100 degrees Celsius.
Required: The heat required to convert water to steam at 100 degrees Celsius.
Step-by-step explanation:
Water is the liquid state while steam is the gaseous state.
The liquid state can be converted into a gaseous state by providing energy usually in the form of heat.
The energy provided to the liquid state is used in two forms before changing into a gaseous state.
The energy provided to the liquid is used to increase the temperature till the boiling point.
Once the temperature reaches the boiling point, the temperature does not increase even on providing energy till the state is completely changed.
This is known as latent heat of vaporization.
The latent heat of vaporization of water is L = 540 cal/g
The heat needed to convert the water to steam at 100 degrees Celsius can be calculated as
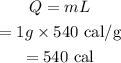
Final Answer: The heat required to convert 1 g of water to steam is 540 cal.