Answer:
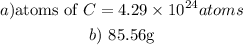
Explanations:
a) Given the following parameter from the question
Mole of carbon-12 = 7.13 moles
Using the conversion rate
1 mole = 6.02 * 10²³ atoms
Determine the atoms of carbon-12:
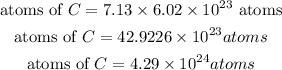
b) In order to determine the grams of carbon-12, the expression below will be used:
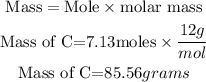
Hence the number of grams the carbon-12 weigh is 85.56 grams