C) Reaction 2 is endothermic as energy is gained by the reaction; the potential energy of the reactants is lower than that of the products therefore energy is absorbed. Reaction 1 is an exothermic reaction meaning energy is released; the potential energy of the reactant is higher than that of the products and so energy is released.
D) All chemical reactions need energy to get started, this energy is called activation energy. In order for a reaction to start the molecules must collide with each other and so they must be in constant motion, this movement requires energy.
E) Not every collision between reacting particles lead to product formation. For product formation from reactants to occur then the collisions must be of sufficient energy and the right orientation. This is not always the case and so not all collisions lead to product formation.
F) The rate of a chemical reaction for a given reaction below is:
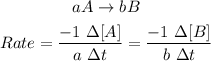
G) Food stored in a refrigerator can stay fresh for a long period of time than food store at room temperature because the lower the temperature the less energy the molecules will have to collide and so the food is kept fresh.