ANSWER
The number of moles of the gas is 0.39 moles
Step-by-step explanation
Given that
The volume of the cylinder is 3L
The temperature of the gas is 350K
The pressure of the gas is 3.75 atm
Follow the steps below
Assume the gas behaves like an ideal gas
The ideal gas equation is given as
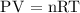

Therefore, the number of moles of the gas is 0.39 moles