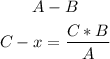Answer:
4 moles of Fe can be made from 6 moles of H2.
Step-by-step explanation:
1st) It is necessary to write the balanced chemical equation:
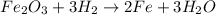
Now we know that 1 mol of Fe2O3 reacts with 3 moles of H2 to produce 2 moles of Fe and 3 moles of H2O.
2nd) With the stoichiometry of the balanced equation, we can calculate the moles of iron that can be made from 6 moles of H2. We can use a mathematical rule of three to solve it:
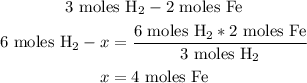
So, 4 moles of Fe can be made from 6 moles of H2.
How to solve a problem with the mathematical rule of three:
In the mathematical rule of three we write, in the first part, the relation between H2 and Fe that we already know from the stoichiometry of the balanced euqation. Then we write the moles that we have, to find the moles that we need to know.
We can write the mathematical rule of three thinking like this:
We know from the balanced equation that 3 moles of H2 can make 2 moles of Fe (that's what we write in the first part of the equation), so the 6 moles of H2 that we have are going to be made from x (that's the second part of the equation). We write x because we don't know the number.
To solve the mathematical rule of three, we have to always multiply C by B, and then divided by A: