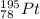Many elements in their atoms can have the same number of protons but vary in the number of neutrons, because these particles have no charge do not significantly affect the element but form what are called isotopes. The representation of an isotope is described in the following figure:
The atomic mass is the sum of the neutrons plus the protons. And the atomic number corresponds to the number of protons.
In this case, for platinum, we have 78 as the atomic number.
So, the mass number will be 78 protons + 117 neutrons = 195.
The isotope symbol will be: