To convert the given amount of moles to mass, we have to use the molar mass of each of the compounds.
BaSO₄ has a molar mass of 233.38g/mol. Use this as a conversion factor to find the mass of 3.95 moles of BaSO₄:
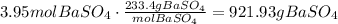
3.95mol BaSO₄ are 921.93g of BaSO₄ .
CuCl₂ has a molar mass of 134.45g/mol. Use this as a conversion factor, just as we did with BaSO₄:
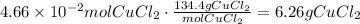
4.66x10^-2mol CuCl₂ are 6.26g of CuCl₂.