Answer: there are 752.6g of AgNO3 in 4.43 moles of this compound
Step-by-step explanation:
The question requires us to calculate the mass that corresponds to 4.43 moles of silver nitrate (AgNO3).
We can solve this problem using the molar mass of AgNO3, which will give us how many grams of the compound are in 1 mol of the compound.
The atomic masses of Ag, N and O are 107.9, 14.01 and 15.99 u, respectively. Thus, the molar mass of AgNO3 can be calculated as:
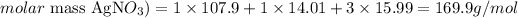
Therefore, the molar mass of AgNO3 is 169.9 g /mol and we now we know that there are 169.9g of AgNO3 in each mol of this compound. Using this information, we can write:
1 mol AgNO3 ---------------------- 169.9g AgNO3
4.43 mol AgNO3 ----------------- x
Solving for x, we'll have:
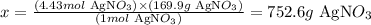
Therefore, there are 752.6g of AgNO3 in 4.43 moles of this compound.