Explanation:
Firstly, we need to define the ionic radius
The ionic radius is the distance between the nucleus and the electron in the outermost shell of an ion.
Recall, that the ionic radius decreases across the period and increases down the group
Given that the atomic number of Potassium is 19 and the atomic number of sodium is 11
The next thing is to write the electronic configuration of both elements
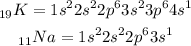
According to the electronic configuration of sodium and potassium, we will observe that sodium belongs to period 3 and potassium belongs to period 4
This implies that Sodium has 3 shells and potassium has 4 shells. Hence, the ionic radius of potassium is larger than the ionic radius of sodium.
PART B
The electronegativity difference explains which bond is more ionic. Due to the structure of potassium and sodium, potassium chloride (KCl) has a higher electronegativity and the size of potassium (K) is greater than sodium (Na) atom. Hence, (K is more electrostatic than Na).
Therefore, KCl has stronger bonding than NaCl
The nucleus contains protons and neutrons while the electron revolves around the shell.
Recall that, both sodium and potassium are alkali metals and have the same chemical properties
The electronegativity of Potassium = 0.82
The electronegativity of sodium = 0.93
The electronegativity of chlorine = 3.16
The electronegativity difference of Nacl = 3.16 - 0.93
The electronegativity difference of NaCl = 2.23
The electronegativity difference of KCl = 3.16 - 0.82
The electronegativity difference of KCl = 2.34
Hence, the electronegativity difference of KCl is higher than the NaCl
NaCl has stronger bonding than KCl due to greater lattice energy
The decrease in the size of atom results in an increase in lattice energy. The size of a sodium atom is small compared to the size of a potassium atom because sodium has three shells and potassium has 4 shells. A decrease in the size of an atom leads to an increase in lattice energy. Hence, stronger bonding is a function of lattice energy.
Therefore, NaCl has stronger bonding than KCl