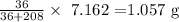Answer:
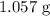
Step-by-step explanation:
Here, we want to know the mass of water that would be lost
To get this, we have to divide the molar mass of water, by the molar mass of the hydrate and multiply by the given mass
The molar mass of water in the hydrate is 2(18 g/mol = 36g/mol). 1 mole of water has a mass of 36 g
The molar mass of the anhydrous part is 208 g/mol
Thus, we have the mass as: