1) What are oxidation and reduction?
Oxidation: This is the loss of electrons. The oxidation number goes up.
Reduction: This is the gain of electrons. The oxidation number goes down.
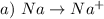
The oxidation number of Sodium changes from 0 to +1.
This is an oxidation.
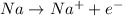
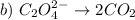
Let's analyze C2O4 (2-). The oxidation number of Oxigen is usually -2 and the charge of the molecule is 2-. The total negative charge is 8-. So, there must be a +6 charge in total. Since there are two carbons, each carbon has an oxidation number of +3.
CO2. The oxidation number of Oxigen is usually -2 and the charge of the molecule is 2-. The total negative charge is 4-. So, there must be a +4 charge in total. Since there are two carbons, each carbon has an oxidation number of +2.
This is a reduction.
----
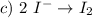
The oxidation number of an ion is the ion charge. So, the oxidation number of I (-) is -1. The oxidation number of an element is 0.
This is an oxidation.
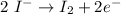
----
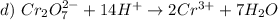
The oxidation number of Oxigen is usually -2 and the charge of the molecule is 2-. The total negative charge is -14. So, there must be a +12 charge in total. Since we have two Cr, each of those has a +6 charge. The oxidation number of monoatomic ions is the ion charge. So, in the products, the oxidation number of Cr is +3.
This is a reduction.
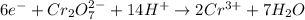
.