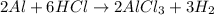Answer
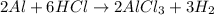
Step-by-step explanation
This reaction between a metal and an acid typically results in a salt and the release of hydrogen gas. When aluminum reacts with hydrochloric acid, the reaction produce aluminum chloride and hydrogen gas. Hence, the balanced equation of compound F when dry hydrochloric acid passes through heated aluminium powder is: