Answer:
110 minutes.
Step-by-step explanation:
Let's see the formula of half-life:
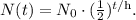
Where N(t) is the quantity remaining, N₀ is the initial quantity, t is time, and h is the half-life.
The problem is telling us that there is 10 % remaining material after 366 minutes. This 10 % corresponds to the division of the quantity remaining and the initial quantity, i.e. N(t)/N₀, so the formula is:
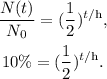
Remember that 10 % in decimals is the same that 0.10:
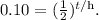
So if we replace the time (in minutes), we will obtain:
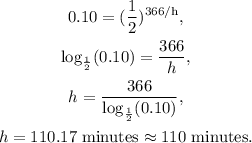
The answer is that the half-life of the radioactive nucleus of the material is 110 minutes.