ANSWER
Step-by-step explanation
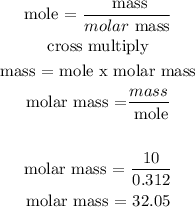
Given that
The mass of the gas is 10.0g
The volume of the gas is 7.69L
The pressure of the gas is 1.00 atm
The temperature of the gas is 27 degrees Celcius
Follow the steps below to find the molar mass of the gas
Step1; Assume the gas is an ideal gas
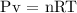
Step 2; Find the number of mole of the gas using the equation above
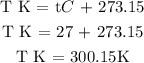

Step 3; find the molar mass of the sample