Answer:
3. Moles.
Step-by-step explanation:
Let's see an example of the gas law formula:
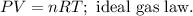
P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is the temperature on Kelvin scale.
The quantity of a gas that can be measured in this calculation would be the number of moles (n). This amount can come from a given mass with its molar mass to obtain moles.
The answer would be 3. Moles.