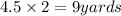Given:
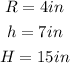
find: volume of the given figure.
Step-by-step explanation: volume of the composite solid=volume of cone + volume of cylinder .
we know volume of cone is
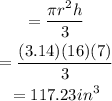
and the volume of the cylinder is
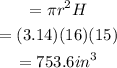
Hence the volume of the composite solid is
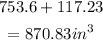
(a) given: the surface area of the sphere is 81pi square yards.
find: the diameter of the sphere.
Explanation: we know that the surface area of the sphere is
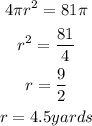
Hence the diameter is equal to twice of the radius