Determine the molecular formula.
Procedure:
1) You know that this compound's percent composition is as follows
Molar mass = 45 g/mol
C => 79.9%
H => 20.1%
It means:
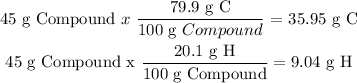
2) Now you have to use the atomic mass in grams of C and H, and determine how many moles of each you need in one mole of your compound.
Atomic masses from the periodic table:
C= 12.01 g/mol
H=1.00 g/mol
For C:
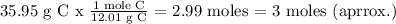
For H:
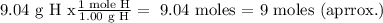
Now our molecular formula:
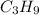
(Note: I'm going to skip the empirical formula, I will get directly the molar mass)