Answer:
0.825 moles of CaSO₄ is produced
Step-by-step explanation:
The given equation of reaction is:
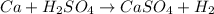
Mass of Ca = 33.0 g
Molar mass of Ca = 40 g/mol
Number of moles of Ca = Mass/Molar Mass
Number of moles of Ca = 33/40
Number of moles of Ca = 0.825 moles
From the equation:
1 mole of ca produced 1 mole of CaSO₄
0.825 moles of Ca will produce 0.825 moles of CaSO₄
Therefore, 0.825 moles of CaSO₄ is produced