According to our question, we need to find the mass of Ga2O3, that can be prepared from 2.14 x 10^24 molecules of O2.
Step 1) It says: mol O2/a O2 molecules. To find "a", we need to remember this:
1 mol of any substance = 6.022x10^23 formula units (atoms, molecules, ions, etc), in this case, we have molecules of O2
So, 1 mol O2 = 6.022x10^23 O2 molecules. Therefore:
a = 6.022x10^23
------------------------------------------------------------------------------
Step 2) b mol Ga2O3/ c mol O2
To know b and c, you must pay attention to their relation in the reaction, and this is (balanced reaction always) 2 moles Ga2O3 / 3 moles O2
So, b = 2 and c = 3
------------------------------------------------------------------------------
Step 3) d g Ga2O3 / mol Ga2O3
For d, you need the molar mass of Ga2O3 (use the periodic table),
187.4 g/mol
So, 1 mol of Ga2O3 = 187.4 g, then 187.4 g Ga2O3/ mol Ga2O3
Therefore, d = 187.4 g
------------------------------------------------------------------------------
At last, we gather everything, and do the math:
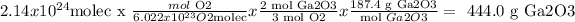
Answer: e = 444.0 g Ga2O3