Step 1 - Understand the behavior of an electrolyte
An electrolyte is a substance that, when dissolved in water, makes a solution capable of conducting electricity. Generally speaking, electrolytes are water-soluble compounds which produce free-ions in solution, such as salts, acids an bases:
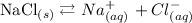
Water does not conduct electricity by itself, because it do not have free ions. Only a solution can produce free-ions: that's why we need electrolytes. The greater the number of free-ions produced, the greater will be the conducvity.
We can measure the conductivity by attaching a light bulb to the circuit. The brighter the light, the stronger the electrolyte.
Step 2 - Determining the weak electrolyte
Since the only substance that do not turn the light on is C, we can conclude that all other substances are electrolytes. Note that B produces only a dimly light, which means he is the weak electrolyte.