Answer:
1.35g of H20 will be produced.
Step-by-step explanation:
1st) It is necessary to write the balanced chemical equation:
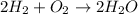
Now we know that 2 moles of H2 gas react with 1 mol of O2 gas to produce 2 moles of H2O.
2nd) With the molar mass of H2 (2g/mol) and O2 (32g/mol) we can find the limiting reactant. The limiting reactant is the one that will run out first and will not allow the reaction to continue:
• H2:
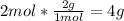
• O2:
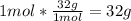
Now we know that 4g of H2 needs 32g of O2 to react properly.
With a mathematical rule of three we can calculate the the grams of each compound using 9.5g of H2:
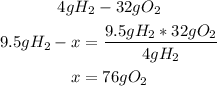
The 9.5g of H2 will need 76g of O2 to react properly, but we only have 1.2g of O2, so O2 is the limiting reactant.
3rd) Finally, using the 1.2g of the limiting reactant (O2), we can calculate the mass of H2O that will be produced:
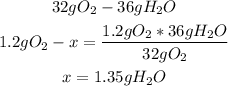
So, 1.35g of H2O will be produced.