We have two states of helium gas. Both states tell us that they happen at constant pressure, we will also assume the ballon has no input or output of matter, therefore the moles will also remain constant.
Therefore, we have a change in temperature and volume at constant pressure and constant moles. If the gas behaves like an ideal gas, we can apply Charles's law which is described by the following equation:
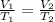
Where,
V1 is the initial volume = 16.8L
T1 is the initial temperature =15°C=288.15K
T2 is the final temperature=-15°C=258.15K
V2 is the final volume, in liters.
Now we clear V2 and replace the known data:
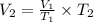
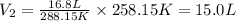
We see that the final volume is lower than the initial one, this is because the temperature is proportional to the volume. If the temperature decreases, the volume also decreases, and if the temperature increases, the volume also increases.
Answer: The volume of the balloon at the final stage is 15.0L