Step 1 - What do we need in order to get fire?
Fire is the result of a chemical reaction. In order to get fire, we need two things: a fuel (usually organic matter, but pretty much anything will do) and an oxidizing agent (which is usually oxygen, but not only).
We could represent it generally thus as:
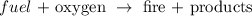
Among the products we can find some gases and ashes, for example. In our example, therefore, the leaves are the fuel, whereas oxygen (in the air) is the oxidizing agent.
Step 2 - How to avoid fire?
Avoiding fire is pretty simple (at least theoretically): just stop the reaction. We can do it by removing the fuel or the oxidizing agent, or by preventing the contact between them.
When we throw a blanket over the pile of leaves, we are preventing its further contact with oxygen. Therefore, the only available oxygen will be the amount of oxygen bellow the blanket. Once it is all consumed, the reaction stops. No fire.
Step 3- Determining the limiting reactant
As the reaction stops when the oxygen is over, this is the limiting reactant in this reaction.