Answer:
A. Fe
Step-by-step explanation:
1st) It is necessary to balance the chemical reaction:
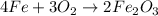
From the balanced reaction we know that 4 moles of Fe react with 3 moles of O2 to produce 2 moles of Fe2O3. We can convert moles into grams using the molar mass of Fe (56g/mol), O2 (32g/mol) and Fe2O3 (159.7g/mol), and we can see that 224g of Fe react with 96g of O2 to produce 319.4g of Fe2O3.
2nd) We can identify the limiting reagent using the stoichiometry of the reaction and the given values of iron (227.8g) and oxygen (128g):
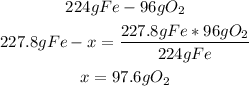
Now we can see that 227.8g of Fe need 97.6g of O2 to react properly, but we have 128g of O2, so the amount of O2 is in excess.
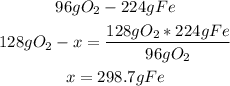
Finally, we can see that 128g of O2 need 298.7g of Fe to react properly, but we only have 227.8g of iron, so Fe is the limiting reagent.