We have to know first the total mass of the solution, so we sum all the mass given:
Water: 28.5 g
Acetic acid: 95.6 g
Acetone: 24 g
Total mass: 28.5 g + 95.6 g + 24 g = 148.1 g
Now, the percent by mass is defined like:
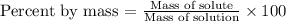
Then we divide each solute by the total mass of the solution and multiply by 100:
Water:
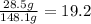
Acetic acid:
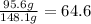
Acetone:
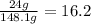
So, the answer will be:
Water mass 19.2%
Acetic acid mass 64.6%
Acetone mass 16.2%